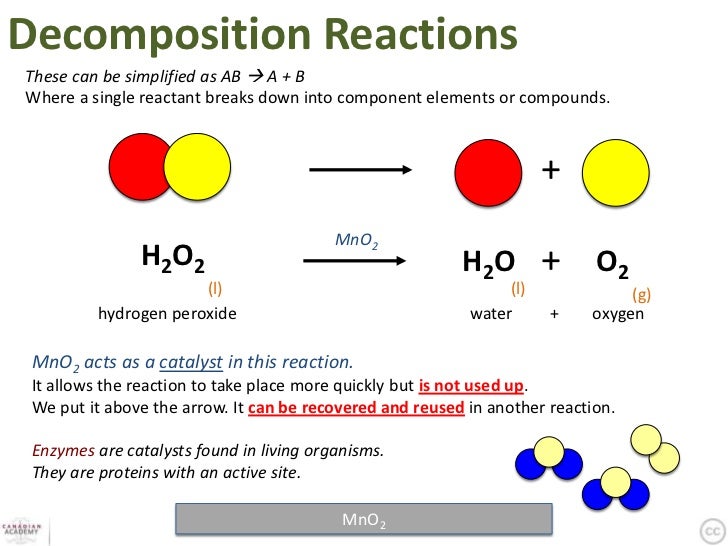
Decomposition Of Hydrogen Peroxide
The decomposition of hydrogen peroxide by catalase is regarded as involving two reactions, namely, the catalytic decomposition of hydrogen peroxide, which is a maximum at the optimum pH 6.8 to 7.0, and the 'induced inactivation' of catalase by the 'nascent' oxygen produced by the hydrogen peroxide and still adhering to the catalase surface. Catalase acts as the catalyzing enzyme in the decomposition of hydrogen peroxide. Nearly all living things possess catalase, including us! This enzyme, like many others, aids in the decomposition of one substance into another. Catalase decomposes, or breaks down, hydrogen peroxide into water and oxygen.
Firstly, Hydrogen peroxide is partly oxdized to Oxygen, and the other part is reduced to Water, it is so called a Disproportionation reaction.Secondly, there are Two reaction in the process actually, one is reaction between KMnO4 and H2O2, which non-catalytic decomposition. Another reaction is H2O2 react with MnO2 which produced in the reaction of KMnO4, and this is the catalytic decomposition.And the reason I didn't balance the equation is simple, the equation have many solution, which means the most probably reaction is related to energetics and some other variable, so this equation cannot be balanced by simple maths. I'm doing a project on the effect of pH on the oxidizing power of permanganate ions on hydrogen peroxide, My pH range is 0-7. (For pH 0-3 I used Hcl and pH 4-7 I prepared buffer solutions) and I'm comparing their initial rates (The rate at which oxygen is formed) and I found ou that the initial rate of reaction increased from pH 0-3 and it decreased from pH 4-7 but I couldn't find any theoretical evidence to support my findings, I was wondering if you could help me with some sources?
Hoi4 manpower by country. One of the keys in this situation is to make the most of the manpower you have. I usually play as Holland, they have pretty low frickin manpower no matter which recruitment setting I use. So, do you really need LOTS of infantry when you could get a lot more tanks or fighters for the same amount of manpower?
Thank you in advance.
Decomposition Of Hydrogen Peroxide Enthalpy
We have investigated the reactions of H 2O 2 with Fe 2O 3, CuO, HfO 2, CeO 2 and Gd 2O 3 in aqueous solution. The reactions rate constants at room temperature were determined. From the temperature dependence of the rate constants we extracted the Arrhenius parameters and the standard enthalpies of activation for the reactions.
In addition, we studied the dynamics of formation of the intermediate species formed during decomposition of H 2O 2, the HO radical. The kinetic data for H 2O 2 reactivity and the yields of hydroxyl radical formation differ considerably between many of the materials studied. We compared the energetic and mechanistic data obtained in this work with literature data for a set of nine oxides in total. The Arrhenius pre-exponential factors normalized to surface area for the decomposition of H 2O 2 vary by nine orders of magnitude for some of the oxides investigated. This indicates that the surfaces of the oxides have very different catalytic capacity towards the decomposition of H 2O 2. The standard enthalpies of activation for H 2O 2 decomposition vary between 30 and 73 kJ mol −1, revealing also differences in the catalytic efficiency for the different materials. The mechanistic study consists of quantifying the HO radical scavenged by tris(hydroxymethyl)aminomethane (Tris) during the course of the decomposition of H 2O 2 for the whole set of oxides.
Decomposition Of Hydrogen Peroxide With Potassium Iodide
The yields and dynamics of scavenging of HO. differ considerably between the oxides analyzed. Surprisingly, the time-independent plots of the amount of HO scavenged as a function of the conversion of H 2O 2 reveals that during the decomposition of H 2O 2 there are turnover points where the amount of HO scavenged by Tris suffers a sudden increase.
The location of these points and the curvatures of the plots at the near-neighbours is considerably different for the different materials.